Introduction to Research Topics
Molecular Probe Synthesis ▪ Molecular Imaging ▪ Functional Molecular Chemistry ▪ Organometallic Chemistry
Research Overview
Synthesis of multifunctional molecular probes for high-level bioimaging
The Kondo Laboratory participates in “Innovative Techno-Hub for Integrated Medical Bio-imaging” (Kyoto University–Canon joint research project, with the participation of Otsuka Pharmaceutical as a collaborating organization (http://ckpj.t.kyoto-u.ac.jp/) of the MEXT’s “Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program” since 2012. We undertake industry–university joint research in the field of engineering-based medicine with a focus on innovations in minimally invasive magnetic resonance imaging (MRI) and optical imaging and promote the formation of a hub for research and teaching in this field. The Kondo Laboratory also participates in the “Last 5X Innovation R&D Center for a Smart, Happy, and Resilient Society” of the Center of Innovation Science and Technology based Radical Innovation and Entrepreneurship Program (COI STREAM) and “Training Program of Leaders for Integrated Medical System for Fruitful Healthy-Longevity Society” (LIMS) of the Program for Leading Graduate Schools. We produce scientists with problem-solving capability and leaders who flourish in the rapidly expanding field of medical engineering and who will battle the pressing issues mounting in Japan’s “super-aging society.”
Specifically, to achieve high-level bioimaging that will greatly contribute to improvements in early disease detection and diagnostic accuracy, the Kondo Laboratory conducts research to design and synthesize revolutionary “molecular probes” with the function of delivery and accumulation at a specific disease site such as cancer as well as the ability to produce the magnetic and ultrasound signals required in imaging (Fig. 1). The laboratory also conducts studies to achieve these functions and to assess their efficacy through cellular and animal experiments .
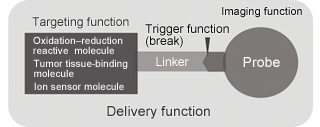
Fig. 1 Schematic diagram of molecular probe
- 1)
- Synthesis and functional/biokinetic assessment of a chiral dendrimer–triamine-coordinated Gd contrast agent for highly sensitive MRI
The Kondo Laboratory designed and synthesized a novel cyclic triamine (triazacycle) ligand by introducing chirality into a dendrimer, a manmade tree-like polymer of a single molecular weight with a high-level structure of regular branches growing outwards from a core, and successfully created a novel Gd MRI contrast agent in which it is coordinated (Fig. 2). By assessing the MRI contrast capability of this Gd MRI contrast agent using a 7T MRI machine for small experimental animals, we demonstrated that it exhibits a relaxivity (r1) of one order of magnitude higher than Magnevist®, an agent currently in clinical use (reported throughout Japan by NHK , Kyodo News Service, various newspapers, and Kyoto University website news release). Moreover, through small-mass measurement using a liquid-crystal oscillator microbalance (QCM) and in vivo animal experiments (vascular contrast, etc.) by using the 7T MRI machine, we demonstrated that the contrast agent, whose absolute configuration at the chiral center is S, exhibits a higher blood retention than the contrast agent whose absolute configuration is R.
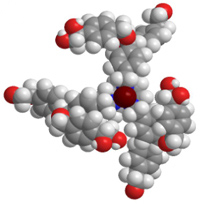
Fig. 2 Novel chiral dendrimer–triamine-coordinated Gd MRI contrast agent
- 2)
- Development and functional assessment of photoacoustic-magnetic resonance (PA-MR) dual imaging probe
Photoacoustic imaging is expected to be the next-generation diagnostic imaging method. At the Kondo Laboratory, we are currently developing a PA-MR dual imaging probe capable of simultaneous PA imaging and conventional MRI. More specifically, we have efficiently synthesized Gd2O3 nanoparticles with a controlled particle size through surface modification using gelatin with excellent biocompatibility and have demonstrated their utility as a PA-MR dual imaging probe (Fig. 3). This technique is also applicable to the synthesis of other paramagnetic metal oxide nanoparticles; we are currently developing an efficient method for synthesizing MnO and CoO nanoparticles using sugars and polyethylene glycol as the surface-modifying agents and assessing their functions. In this study, we are developing a safe PA-MR dual imaging probe made of only a paramagnetic metal oxide rather than using fluorochrome or gold nanoparticles with the goal of improving early disease detection and diagnostic accuracy through two independent image types.
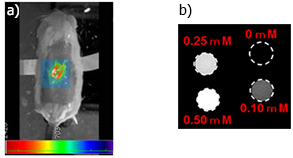
Fig. 3 a)Photoacoustic image after the subcutaneous administration of gelatin-modified Gd2O3 nanoparticles in mouse
b)MRI image of gelatin-modified Gd2O3 nanoparticle aqueous dispersion
- 3)
- Construction of a multifunctional molecular probe biodelivery system
In general, compounds administered within the body are quickly eliminated from the body through metabolism by the liver and macrophages and filtration in the renal glomerulus. Therefore, substances that must be used in the body over a long period, such as drugs and labeled compounds, require a method for supplying them to a target site (tumor cells, etc.) whose environment (pH, oxygen concentration, surface antigens, sugar chain structure) can be detected. Such a technique is called the drug delivery system (DDS). By applying this concept to molecular probes, improved accumulation efficiency at a specific site such as a tumor and improved blood retention are expected (Fig. 4). By creating DDSs using molecular probes and diagnostic agents with low toxicity and high sensitivity, we are currently conducting research for creating a novel multifunctional molecular imaging probe as a tool for obtaining new biological data.
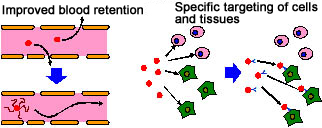
Fig. 4 Biokinetic control image of a molecular probe through a DDS
- 4)
- Development of a revolutionary molecular target for MRI technique using multiple-resonance NMR
(Joint research with Prof. Shirakawa, Associate Prof. Tochio (Department of Molecular Engineering), Prof. Matsuda (Graduate School of Informatics), Prof. Aoyama (Professor Emeritus, Kyoto University), and Prof. Sando (University of Tokyo))
At the Kondo Laboratory, we are developing a next-generation MRI technique that uses multiple-resonance NMR, which has been widely used in the structural analysis of proteins. Multiple-resonance NMR is a method for observing specific bonds within a molecule (for example, only the 1H bond in the 1H-13C-15N system). In this method, the magnetization transfer (signal information transfer) of an NMR signal of the 1H nucleus to the adjacent NMR-active nuclei (13C or 15N nucleus) is carried out, the magnetization is then returned, and the signal of the 1H nucleus is finally detected. At our laboratory, we have developed a phosphorylcholine polymer (13C/15N-PMPC) probe consisting of a phosphorylcholine skeleton, which is the part of cell membrane lipids, labeled with 13C and 15N nuclei as a stable isotope-labeled probe, enabling molecular MRI using a multiple-resonance NMR. By administering tumor-bearing mice, we have successfully achieved highly selective imaging of only tumor sites at which the 13C/15N-PMPC probe has been accumulated. We also demonstrated that this probe does not accumulate in general organs such as the liver, kidneys, and brain (Fig. 5, reported throughout Japan by Nihon Keizai Shimbun and Kyoto University website news release). Thus, molecular MRI using this stable isotope-labeled probe that accumulates highly selectively only at the tumor sites is a next-generation diagnostic imaging method with sensitivity and selectivity rivaling those of positron emission tomography (PET) and unlike PET is capable of early diagnosis and repeated examinations of cancer on the order of several millimeters with zero radiation exposure. We expect that it will serve as a trigger for innovation in the field of medicine.
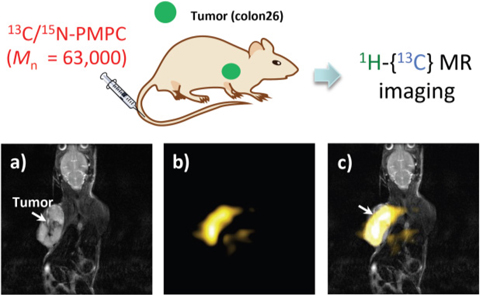
Fig. 5 In vivo MRI image of mouse administered 13C/15N-PMPC probe
a: Conventional 1H MRI image (T2-weighted image). b: Molecular MRI image (1H-{13C} double-resonance MRI image). c: Superimposition of image (a) and image (b).
- 1) Development of a highly selective linear cotrimerization reaction of three different alkenes using a Ru complex catalyst
The development of new catalytic organic synthesis reactions based on organometallic chemistry is a key research topic in synthetic organic chemistry, and highly versatile catalytic reactions have become critical tools in precision synthesis such as molecular probe synthesis. For example, in the synthesis of the chiral dendrimer–triamine ligand described above, we use the asymmetric dihydroxylation (AD) reaction of alkenes using an Os catalyst developed by the 2001 Nobel chemistry laureate Prof. K. Barry Sharpless. The Kondo Laboratory is the world leader in research on Ru complex catalysts. For example, using our independently developed zero-valence Ru catalyst, Ru(η6-1,3,5-cyclooctatriene)(η2-dimethyl fumarate)2, discrimination among three different alkenes of low electronic state (N-vinylamide, an acrylic acid derivative, and ethylene) became possible, and regio- and stereoselective linear cotrimerization reactions could be achieved (Fig. 6). This research was published in a highly reputed international journal Angewandte Chemie International Edition and appeared on its cover page. The results have also been widely reported in Japan in various newspapers, with a summary published in a news release on the Kyoto University website.
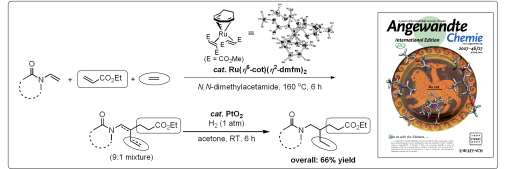
Fig. 6. Highly selective linear cotrimerization reaction of three alkenes using a Ru complex catalyst
- 2) Synthesis, single-crystal X-ray structural analysis, and elucidation of the catalytic function of oxygen-crosslinked four-Ru-nuclei complex
When Ru(η6-1,3,5-cyclooctatriene)(η2-dimethylfumarate)2 complex was reacted with water for activating the water molecule by the organometallic complex catalyst, a new four-Ru-nuclei complex, in which the oxygen atoms were crosslinked, was obtained in high yields. Its structure was elucidated through a single-crystal X-ray structural analysis. We also discovered that this four-Ru-nuclei complex exhibits high catalytic activity in a highly selective oxidation reaction of alcohol with aldehydes or ketones using air as the oxidant. Because the reaction produces only water as the by-product and does not require a strong oxidizing agent such as a heavy-metal oxide or peroxide, it is an environment-friendly clean alcohol oxidation reaction (Fig. 7).
We recently discovered that an oxo-crosslinked four-Ru-nuclei complex exhibits high catalytic activity in the intramolecular dehydrogenation N-heterocyclization reaction of 2-aminophenetyl alcohols and succeeded in developing a highly efficient method for constructing an indole ring that is useful as the basic scaffold of various bioactive substances.
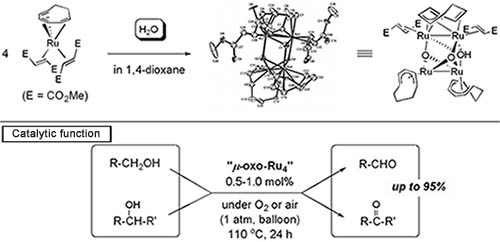
Fig 7. Synthesis and catalytic function of oxo-crosslinked four-Ru-nuclei complex
- 3) Development of an intermolecular [2+2+1] co-adduct cyclization reaction of an isocyanate, an alkyne, and carbon monoxide (CO) using a Ru complex catalyst
The Kondo Laboratory has been developing new methods for synthesizing functional heterocyclic compounds using a key intermediate of “ruthenacycles” containing Ru metals in the ring. For example, using a Ru3(CO)12 catalyst, the intermolecular [2+2+1] co-adduct cyclization reaction of an isocyanate, an alkyne, and CO proceeds well. We have also successfully developed a highly efficient one-pot synthesis method for maleimide derivatives, which are important basic scaffolds of transparent heat-resistant resins, nucleic acid modifiers, etc. (Fig. 8). Furthermore, a series of heterocumulenes can be used in this catalytic reaction, and highly efficient one-pot synthesis of 2-furanone derivatives using ketenes as the starting materials or azolinone derivatives using carbodiimides as the starting materials has become possible.
![Intermolecular [2+2+1] co-adduct cyclization reaction of an isocyanate, an alkyne, and carbon monoxide using Ru3(CO)12 catalyst Intermolecular [2+2+1] co-adduct cyclization reaction of an isocyanate, an alkyne, and carbon monoxide using Ru3(CO)12 catalyst](https://www.abe.ehcc.kyoto-u.ac.jp/wp-content/themes/ku_kondo_en/img/sub/study/study_img10.jpg)
Fig 8. Intermolecular [2+2+1] co-adduct cyclization reaction of an isocyanate, an alkyne, and carbon monoxide using Ru3(CO)12 catalyst



